Just What the Hell is Fire? (Heh heh, Fire!)
 These are a series of articles dedicated to that moment when you stop to think about something simple and common from your everyday life, and realize you have no freaking idea how it works at all. For example, just what the hell is fire, exactly? We’ve all heard the science, we know about combustion, but what exactly is the orangey-yellow stuff I stare at creepily at the campfire? Today let’s discuss just what the hell is fire?
These are a series of articles dedicated to that moment when you stop to think about something simple and common from your everyday life, and realize you have no freaking idea how it works at all. For example, just what the hell is fire, exactly? We’ve all heard the science, we know about combustion, but what exactly is the orangey-yellow stuff I stare at creepily at the campfire? Today let’s discuss just what the hell is fire?
Explanations for exactly what fire is, come in two forms:
1) “Fire is HOT!!! Don’t touch!”
2) “Fire is the rapid exothermic oxidation of combustible material in a gravitational hyperplane differential epistemology. Don’t touch!”
In other words, every explanation I’ve seen is either a little too simple or a little too complex. And yet I still find myself not able to explain exactly what the yellow-orange stuff is that I’m currently carbonizing a marshmallow in. You get a rough idea of what fire is back somewhere around 6th grade, where you vaguely learn about how it uses oxygen. Then maybe you pick up some cool factoids about it later on in chemistry class, such as how rusting could be seen as an extremely slow process of burning. But let’s put that all aside, and get right to the central issue — just what the hell is fire?
More specifically, what is *flame*? I think I’ve got a good idea of what smoke is, and I’ve heard of the concept of “exothermic”. But just exactly what is the orangy glowing stuff? Turns out, fire is just a chemical reaction — it’s happening in a fire pit at the beach instead of a trapezoid-shaped flask in your chemistry lab, but it’s a chemical reaction nonetheless. Speaking of chemistry class, remember “endothermic” and “exothermic” reactions? Some chemical reactions (called endothermic reactions) steal heat from their environment when they occur — you know these by how the test tube literally feels cold in your hand as they proceed. Other reactions release heat as they progress — these exothermic reactions are pretty important to our society, particularly in things like car engines.
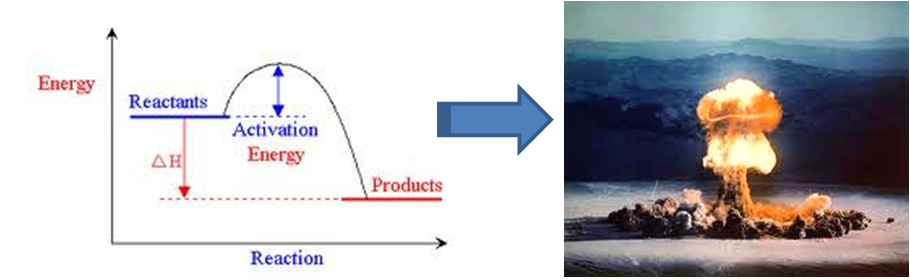
Fire comes from a particular kind of exothermic reaction — a little chemical reaction called combustion is going on, and that reaction releases energy. A lot of energy. That energy obviously gets released as heat, hence “don’t touch!”. Like pretty much all reactions, combustion needs a little bit of input energy to get going, such as the heat generated when you use friction to strike a match. (If you ever took chemistry, you probably saw the little hill-shaped energy profile curve.) But once it gets going, it’s a net exporter of heat, which means it can self-sustain – the heat it releases can be used as the input energy necessary to get more combustion going. As long as you give it fuel and oxygen, it can keep on chugging indefinitely.
OK, enough chemistry — let’s talk about the *flame*. It looks so otherworldly, so different than what other stuff you come across day to day, it’s no wonder we’re fascinated by it. What state of matter is it? If you didn’t mind burning your fingers, could you reach out and pull off a pinch of fire? What kind of *stuff* is it? Is it pure energy?
Turns out the flame is made of the same thing as this website you’re reading — hot gas. The chemical reactions going on down in that log of white pine you threw in the fire pit release various gases, mostly carbon dioxide and water vapor. Plus a lot of heat. So much heat, in fact, that the released gases start glowing, just like a lump of charcoal or hot electric stove burner.
Anything that’s hotter than absolute zero actually *glows* — we’re familiar with it for very hot objects, like a red-hot glowing charcoal lump, but things don’t have to be that hot to still glow. The hotter something is, the higher-wavelength light it emits — that’s why glowing-hot objects go through the progression of red to orange to yellow to white to blue as they get hotter and hotter — you don’t want to touch something red-hot, but you don’t want to even go near something white-hot (or worse, bluish hot). As your red-hot charcoal lump cools off, the reddish glow dims and disappears. But the lump didn’t stop glowing — the glow just dropped down into a longer wavelength of light than your eyes can see. That lump is now glowing brightly in the infrared. If your eyes were capable of seeing in the infrared, you’d see the little lump progress through ever-cooler infrared colors as it cooled off to room temperature. In fact, we as humans are just hot enough to glow in infrared, which is why you see ghostly outlines of people on infrared cameras.
So the gases floating upward from the spot of combustion are heated so much from the chemical reaction that they’re glowing in a wavelength of light we can see with our eyes. The hotter the gas, the whiter / bluer the color — so that’s why the center of the flame looks the whitest, and the tips of the flames (where the gas is the coolest) are also the reddest. Similarly, extremely hot flames (such as from an arc welder) emit light at wavelengths *higher* than we can see, up in the ultraviolet — that’s why welders need to wear masks to look at the flame, to avoid frying their eyeballs in UV light.
So what looks like an isolated spot of dancing glowing stuff that we call “flame”, is really just the hot center of a bigger cloud of gas, where the center is the only part hot enough to glow in the visible range of light. Just beyond the outer edge of a flame, you’ll find more hot gas that is just a smidge too cool to glow in a range of light we can see — but it’s still glowing, in infrared colors that our eyes don’t respond to. What looks to us like a sharply-defined little pocket of glowing crud is really just the center of a larger plume of hot gases, with a full spectrum of colors going from white to yellow to red to infrared as you move out from the candle wick. So the boundary at the edge of the flame is really the boundary between red and infrared — a sharp boundary that exists only in our eyes, not in the flame itself.
While this is kinda cool, I admit it’s a little disappointing to learn that the flame is made of more mundane stuff than I thought. It’s a lot of carbon dioxide and water vapor (which are also in our exhaled breath), heated up enough to start glowing. I dunno about you, but I was expecting to hear that it was made of Magnetized Vortex Plasma or something 1. Seems like something that looks so beautiful and alien ought to be made of something more exotic than really hot coffee breath.
I’ll admit a bit of embarrassment that I couldn’t until now explain precisely what the flame is, despite having a couple degrees in physics. As it turns out, I’m not the only one — after first writing this article, it was pointed out to me that lots of folks have contributed their own explanations to this question. Check these out for more about fire, particularly about the intricacies I didn’t cover (like how the chemical constituents affect the color of the fire, or how exactly a hot atom emits light):
- Alan Alda has been a staunch supporter of science education for years, and created a contest to goad scientists into explaining exactly what fire is. Years ago, an 11 year old Alan asked his science teacher what flame is, and received the disappointing answer, “oxidation”. That teacher might as well have added “go away kid, ya bother me.” Check out his Flame Challenge and the winner’s explanatory video. (Thanks to
@Summer_Ash for pointing this out to me!) - The excellent science educator
@minutephysics has also done one of his excellent Minute Physics videos on fire. Check it out here. (Thanks to@PremiumMichael !).
Footnotes
1 — if you think reading the standard definition of fire is confusing, try reading internet threads debating whether or not a typical candle flame is a plasma or not. You do hear it mentioned that fire is an example of the fourth phase of matter, the epically-named plasma, but the tide of the debate seems to sway against it. Perhaps some really hot flames created using exotic materials might be on the borderline between hot gas and plasmas, but the scented candle you light after destroying the guest bathroom doesn’t generate enough of a wallop to fully ionize gas particles at more than negligible amounts, so it can’t really be called a plasma.


 Follow Timeblimp on Twitter
Follow Timeblimp on Twitter